Comparison of Omics Techniques and Biomarker Types
Get the Resource
Download Article PDFHigh throughput technologies can now capture and measure a huge number of biological molecules within a single human cell or tissue, enabling clearer and more complete views of underlying biology and supporting development of molecularly targeted drug therapies. These molecules are found at the gene, protein, and metabolic level, giving rise to multiple ‘omics’ disciplines.
Each discipline is necessary to build a comprehensive understanding of the molecular activities, interactions, and functional pathways that influence and are involved in human health and disease, and each can be used to identify biomarkers that help elucidate disease risks, predict clinical outcomes, and guide development and dosing of therapeutic interventions.
Below we compare the different types of biomarkers derived from omics studies in more detail, with practical considerations for their discovery and use.
Biomarker Comparison Table: A Closer Look
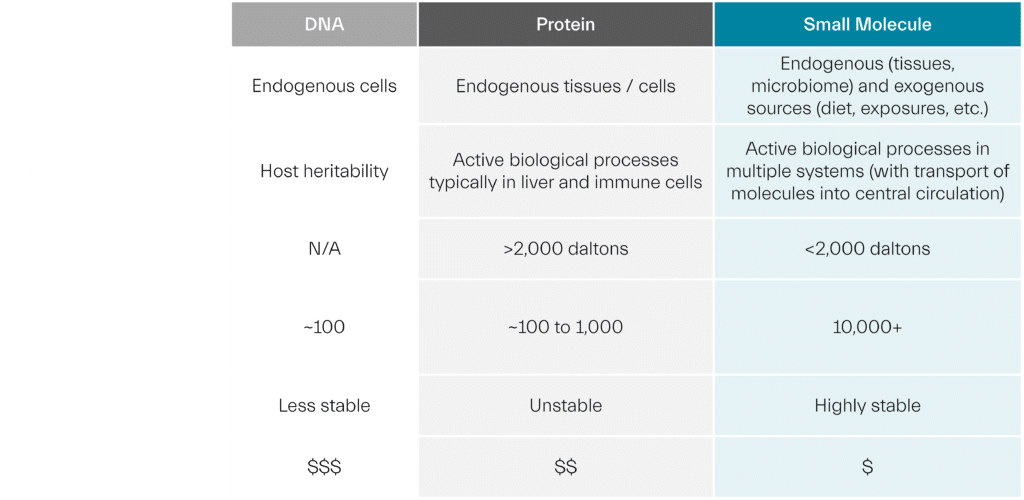
DNA Biomarkers: Informing Host Heritability
A genomic biomarker is a measurable characteristic of DNA that indicates a biological process, pathogenic process, and/or a response to a therapeutic or other intervention. This could be a measurement of the expression, function, or regulation of a gene.
Genome-wide association studies (GWAS) enable rapid discovery of genetic variants that contribute to disease susceptibility and that influence treatment response. DNA biomarkers, including single nucleotide polymorphisms (SNPs), short tandem repeats (STRs), and deletion or insertion sequences, allow us to gauge how much phenotypic variation in a given population is attributable to individual genetic differences, and provide better understanding of the heritable components of disease risk and/or drug response.
This information is extremely valuable to diagnose diseases with genetic links and to align patients with therapies that target their distinct genetic changes. However, genomics only tells part of the story. The risk of acquiring disease is influenced not only by an individual’s genetics, but also by the exposures the person experiences throughout their lifetime – and how those exposures interact with genetics. In fact, studies show that genetic factors are not the major causes of chronic diseases.
Protein Biomarkers: Revealing Cellular Activities
A complementary approach to understand how genomic information is expressed to determine phenotype is through the identification and characterization of cellular gene products – protein biomarkers – that are present, absent, or changed under different environmental, physiological, and pathophysiological conditions.
Proteomics analyzes the properties of proteins expressed by endogenous cells and tissues, including their location, abundance/turnover, post-translational modifications (PTMs), and interaction partners.
Proteins are responsible for cellular function, and protein biomarkers can elucidate protein activity, functional pathways, and protein interactions in organs such as the liver or kidney to guide disease diagnosis and treatment decisions. Proteins, however, generally remain in these tissue beds and are only actively extruded into the circulatory system for specific biological reasons, such as cell death stemming from damage to the organ. Short of invasive tissue extraction, this limits the ability to gain early insight into disease processes occurring in organ systems.
Small Molecule Biomarkers: Providing Predictive Power
To readily gain information about biological processes occurring in real time in particular tissues, we can turn to small molecule biomarkers. These low molecular weight compounds, including polar metabolites, polar lipids, and nonpolar lipids, are small enough to cross cellular and biological membranes to enter into central circulation. The circulating chemistry can be captured via blood to provide information on the processes occurring in and interacting across systems, such as the brain, liver, heart, or muscle, as well as peripheral tissues and tumors.
Small molecule biomarkers are generated from interactions between genes, transcripts, and proteins, and can also be produced by exogenous factors such as diet, physical activity, and environmental exposures. They are therefore uniquely suited to depict phenotype, providing functional readouts from varied sources – what we eat, the medicines we take, the geography we live in, and more – to reveal how non-genetic factors impact health and disease. They provide early insight into mechanisms of disease in individual tissues and are particularly important for understanding target engagement.
Sapient’s Approach: Small Molecule Biomarker Discovery at Scale
Sapient focuses on small molecule biomarker discovery because we have found these biomarkers to hold the most predictive information, providing powerful insight to bridge the gap in understanding of genotype-phenotype relationships.
Small molecules read out active biological processes occurring across multiple systems, and because they pass from local tissues into central circulation (actively or passively), they can be non-invasively captured for greater speed of discovery at lower cost.
In fact, Sapient’s rLC-MS systems have the capacity to profile thousands of samples per day, measuring over 11,000 small molecule biomarkers per sample. Our analysis is untargeted to include capture of uncharacterized compounds, and enables greater scale of discovery: the vast majority of the biomarkers we find are novel. In comparison, proteomics analysis at current high-throughput scale allows for the measurement of hundreds to thousands of (generally well established) protein biomarkers in hundreds to thousands of samples per day.
With small molecules, we can capture information not only on the metabolism of a drug, but direct biomarkers of target engagement, indirect biomarkers of biological effects, and thousands of markers about the host which may influence overall drug responsiveness. However, it is important to remember that these chemical processes do not happen in a vacuum.
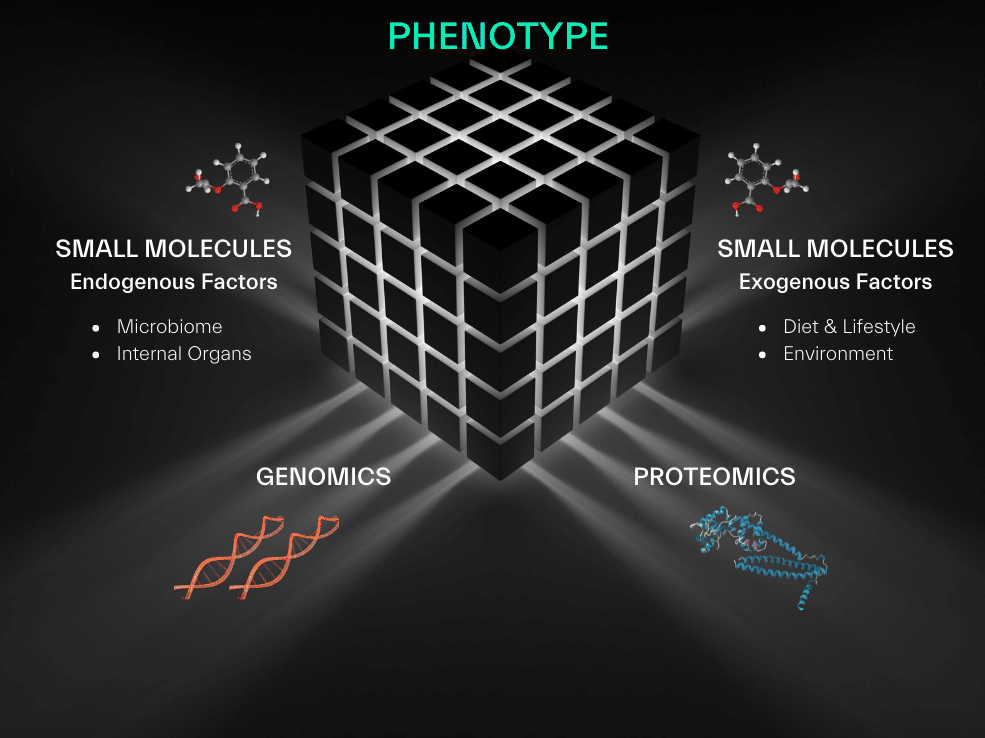
We integrate data generated from our small molecule biomarker discoveries with genomics and proteomics data to build a comprehensive understanding of biological processes, disease mechanisms, and drug actions at the molecular level. Together these insights are catalyzing a new era of efficient drug development and targeted treatments.
Interested in seeing how small molecule biomarkers are being applied in drug pipelines today? Reach out to our team to learn more.