Getting Drug Development Right
The omics revolution has enabled many breakthroughs in our understanding of disease biology, but the productivity of clinical drug development remains historically low. In 2020, the composite success rate of drugs across all therapy areas was only 9.8% – even lower than the 10-year average of 12.9%.
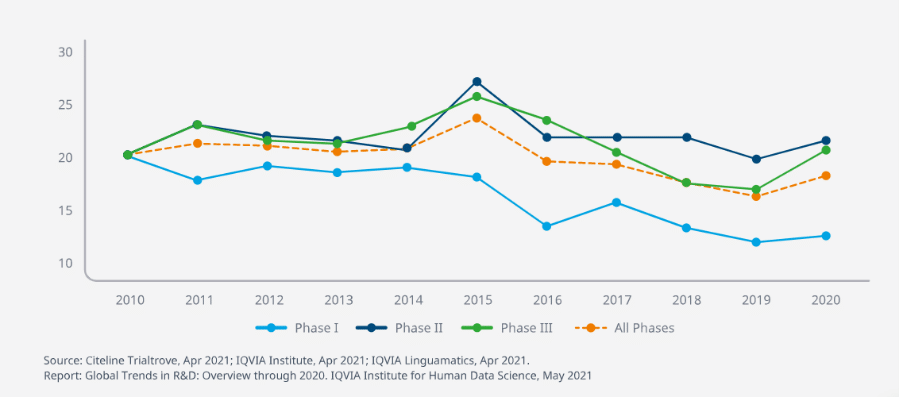
Figure 1. Clinical Development Productivity Index by phase and overall.
Significant inefficiency stems from the late point at which drug efficacy and safety are determined. 41% of drugs that reach Phase III are still likely to fail. With clinical development productivity measured by successful trial outputs compared to the time and money invested to achieve those outputs, these late-stage failures drive down the overall rate dramatically.
So how do we achieve sustainable drug development productivity? The answer becomes clear when you consider the 5 most important determinants of drug success and pipeline quality:
1. The Right Target
Lack of efficacy is a leading cause of failure in clinical trials. The safest and most potent drug candidate will still fail if it is modulating the wrong target, making target selection one of the most important decisions in the drug discovery process. Often, limited knowledge of target biology leaves scientists unable to confidently establish a connection between target and disease, causing projects to progress to expensive proof-of-concept studies before efficacy issues arise.
This is where biomarkers can come in. Used to uncover, select, and validate new targets with a strong link to disease, efficacy biomarkers can instill high confidence in target selection or rapidly invalidate the hypothesis. In fact, a study connected on one large pharma’s pipeline found that 82% of projects that had an associated efficacy biomarker were active or successful in Phase IIa trials, compared to less than 30% of projects without such biomarkers.
2. The Right Mechanism
Once target selection has been confirmed, probability of success can be increased through demonstration that the candidate drug achieves sufficient exposure and pharmacological activity in the target organ. Pharmacodynamic (PD) biomarkers play a role here by providing an integrated understanding of exposure at the site of action, target occupancy, and target expression: known as the ‘Three Pillars of Survival’ that determine the likelihood of a drug candidate progressing from Phase II to Phase III trials.
3. The Right Patient
It has been shown that drug trials with a clear patient stratification plan, which considers the different genetic, molecular, and functional disease profiles of patients, have a higher probability of demonstrating clinical benefit and in turn, transitioning to later-stage trials. Predictive biomarkers are key to patient selection and stratification, as they identify individuals who are most likely to respond to drug exposure – either beneficially or with an adverse effect. This helps to efficiently differentiate the likely outcome of therapeutic intervention within patient populations, and to identify population subsets in which the drug is most effective.
4. The Right Safety
Hand-in-hand with identifying the ‘right patient’ for a drug is establishing an acceptable safety profile that considers benefit vs. risk to the patient. This can be challenging because not all safety signals can be predicted at an early stage, owing to the fact that 17% of Phase III trials fail due to safety. However, the continued development of predictive safety biomarkers will help to close this gap by providing early insight into potential risks – both in the preclinical phases and as the drug transitions to clinical trials.
5. The Right Pharmacometrics
Pharmacometrics brings together data gained through target selection, mechanism assessment, patient selection, and safety profiling to develop quantitative drug, disease, and trial models that aid development and regulatory decision-making. Drug models describe concentration-effect, dose-response, and PK/PD relationships; disease models describe the relationship between biomarkers and clinical outcomes; and trial models inform patient inclusion/exclusion criteria. These analyses require a multi-disciplinary approach – with scientists and statisticians coming together – to effectively integrate knowledge across the development program.
As you can see, biomarkers deliver significant value to transform productivity across the drug development pipeline. They help define the differentiation of a drug as well as its commercial viability by answering questions such as, “what patient populations will it be successful in?” or “how is the target mechanism working differently?” Understanding these key drivers of success helps to make ‘go’/’no-go’ decisions more quickly and brings more transparency to the strengths and risks of the project. Biomarker led drug development brings focus to increase the probability of successful transitions through trial phases and, ultimately, to approval – as shown by the data below:
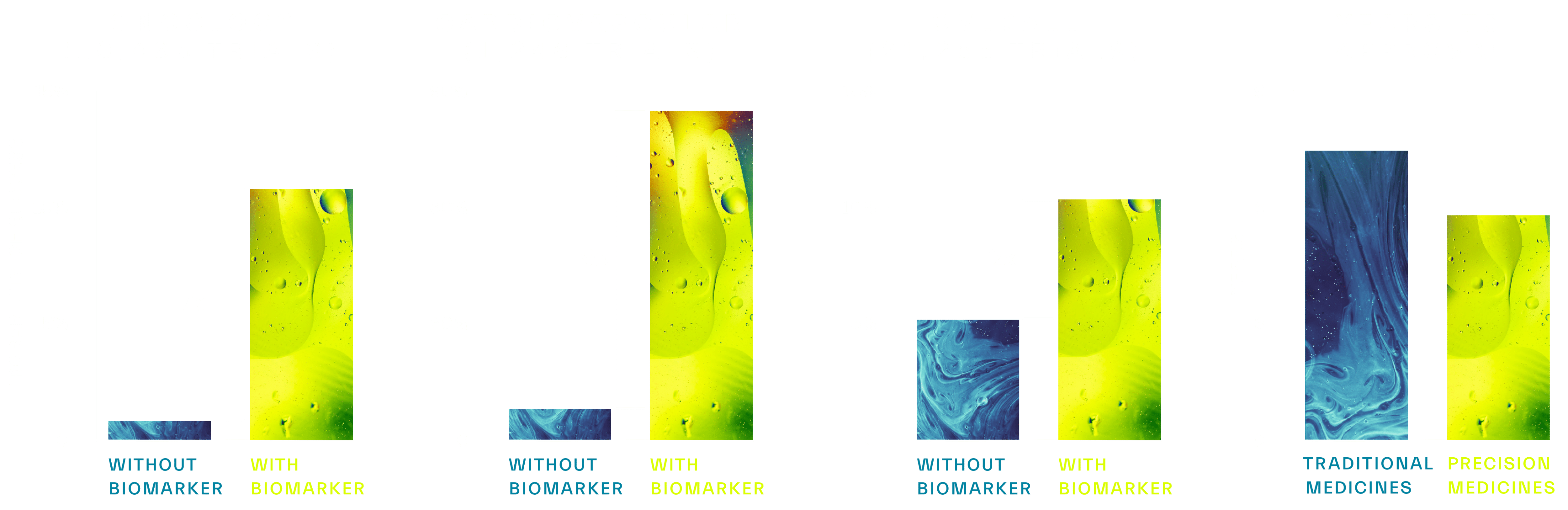
Figure 2. Across >20,000 clinical studies, biomarkers were shown to increase probability of success (POS) of FDA approval by >2-10x.
Want to find the biomarkers that will optimize your own drug development efforts? Reach out to us today to learn how Sapient can help.