Biomarker-Driven Clinical Trials: Enriched with Biology, Evolved for a New Era
Why are large, expensive clinical trials so common? It stems from the challenge of needing to demonstrate treatment efficacy in enough of the patient population despite the fact that a majority of patients are likely to be non-responders. The FDA generally expects at least 35% of patients in a trial to be “responders” in order to advance the drug program to approval, but even that percentage is often difficult to attain.
Much is this is often due to the fact that most diseases are heterogenous in molecular pathogenesis, meaning patients’ treatment responsiveness will also vary. Without a strong understanding of underlying disease biology, a wide net must be cast in the patient selection process to ensure inclusion of a sufficient number of individuals most likely to benefit from treatment. These patients often represent the minority of those enrolled in a clinical trial.
A number of challenges then arise, as larger clinical trials inherently have longer timelines and greater costs due to the need to recruit, enroll, and monitor more patients. A study published by JAMA Internal Medicine found that clinical trials with fewer than 100 patients cost an average of $5.9 million, while those with more than 1,000 patients had an average cost of $77.2 million. Even these are conservative estimates as costs can easily increase to the hundreds of millions depending on therapeutic area. In addition to sponsor-related issues, patient access to needed medicines is delayed. Most concerning, however, is that drugs approved through these large trials are likely to be broadly prescribed, but may be ineffective for as much as two-thirds of patients. Pause on this for a moment: for a patient, the probability of efficacy from many approved drugs is at best no better than the toss of a coin, and often worse. Many patients are likely to absorb all of the risk of taking the drug without the benefits.
These issues illuminate why the FDA has taken a strong interest in efforts aimed at improving patient selection for clinical trials. This is the basis for its recent gudiance on clinical trial enrichment strategies.
The Goal: Pinpoint the Right Patients
The FDA defines enrichment strategies for clinical trials as those intended to increase the efficiency of drug development and support precision medicine by tailoring treatments to patients who will benefit based on clinical laboratory, genomic, and proteomic factors. Instead of treating everyone with the same end pathology in the same way, enrichment strategies aim to stratify patient populations according to the different pathways that contribute to their disease, understanding that the involvement of specific genes, proteins, environmental exposures, and/or molecular alterations that give rise to any one disease may be varied across individuals.
Predictive biomarkers can play a critical role in enabling clinical trial enrichment by providing a biological measurement based on an understanding of the drug’s mechanism of action and the role of the drug target in the pathophysiology of the disease. In this way they can indicate if a patient is likely to respond to a particular drug.
Biomarkers can also be used to characterize disease pathways in a narrow range to decrease interpatient variability. Trial enrollment, therefore, can be restricted to only biomarker-positive patients. Biomarkers can even be used during the trial itself for adaptive enrichment, by identifying markers that indicate if the drug is benefitting all or only a subpopulation of patients. Patient selection for the remainder of the trial can then be restricted to the responsive subpopulation if needed.
This has the potential to give rise to smaller, faster clinical trials, prevent non-responder patients from being exposed to unnecessary drugs and potential adverse events, and help align patients with the most effective therapy so they receive maximum benefit.
Moving the Needle by Changing the Curve
Below is a visualization of the impact that biomarker-driven enrichment strategies can have on the size and effectiveness of a clinical trial. It simulates a typical Phase III trial size, with volunteers split between the control group and those receiving the intervention. While the drug demonstrates adequate efficacy to advance to approval, only 35% of the patients in the intervention group actually responded to the drug. These factors meant that the study needed to enroll 2,000 patients to achieve an acceptable p-value.
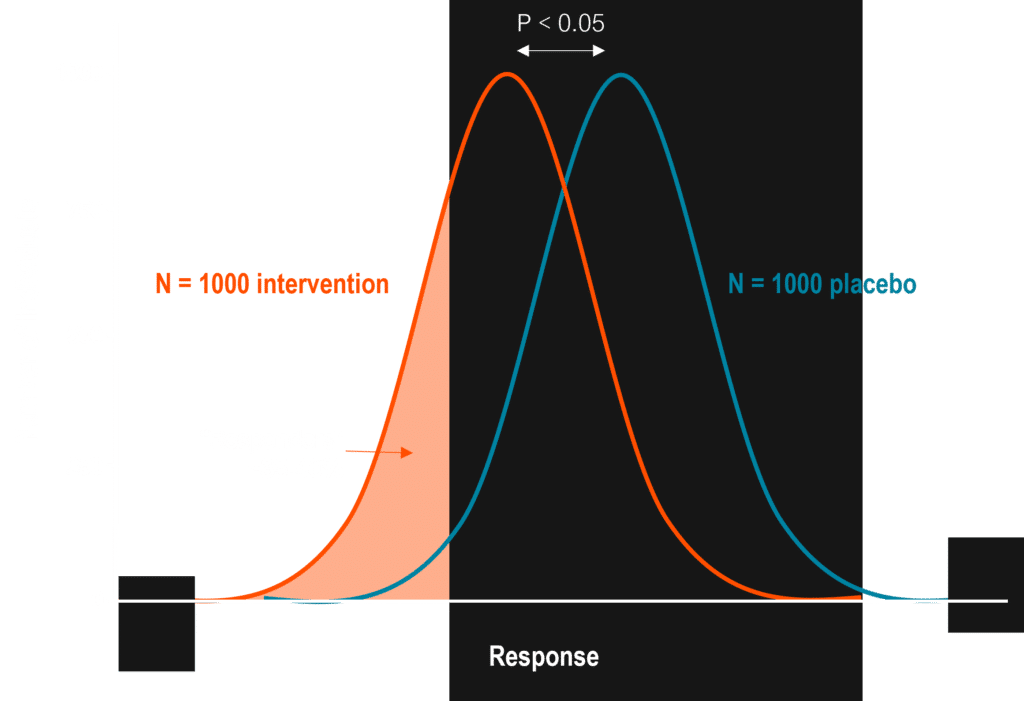
Now, if we enrich the same trial by enrolling only patients that have a biomarker indicating they are likely to respond to the drug therapy, we can significantly lower the overall trial size while at the same time demonstrate maximum benefits for the intervention group.
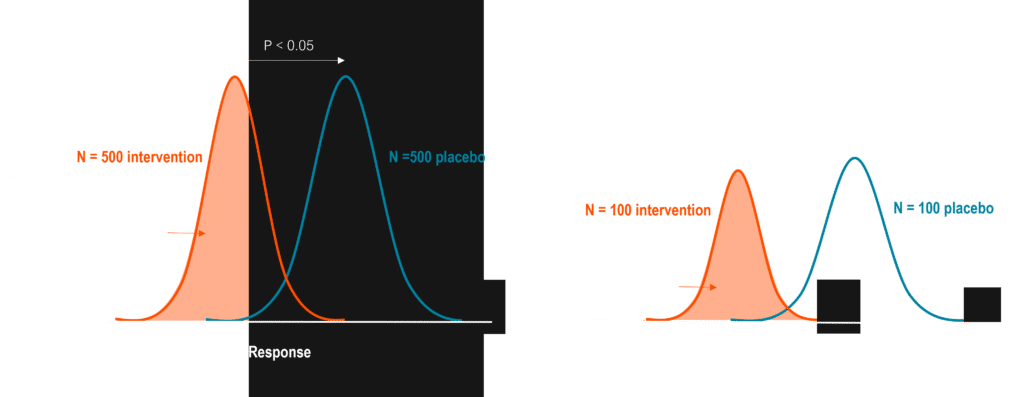
Even a biomarker that enriches the trial to include 70% responders will reduce the trial size by 2x; identifying a perfect diagnostic biomarker can help ensure that only responders will be enrolled in the trial. You can imagine the time and cost savings of reducing a clinical trial’s size by as much as ten-fold – which directly translates to patient benefit as they gain faster access to effective therapies at lower cost.
Enriching Biomarker Discovery to Transform Trial Efficiency
It will be vital to continue expanding our knowledge of underlying mechanisms of disease to achieve the full benefits of clinical trial enrichment. More biomarkers are needed to help stratify heterogenous patient populations and elucidate the varied molecular and pathway alterations that manifest specific diseases. While the advent of next-gen sequencing technologies has rapidly increased the discovery and use of genomic biomarkers, genetics only account for a minority fraction of the many factors that give rise to chronic diseases. The capture of small molecule biomarkers, which provide readouts of human exposures coming from genetics, diet, the environment, toxicants, other drugs, and even internal organs and the microbiome, will help to fill gaps in understanding of disease pathways that produce pathology, and better enable personalization of medicine.
The sheer volume of circulating factors that can be measured, but are yet to be profiled, represents a profound opportunity to identify new specific, sensitive biomarkers to enrich trials and align the right patients with the right therapies. This is the mission of Sapient: to probe deeper into the non-genetic landscape of disease and explore unmapped molecules at a speed and scale that creates rapid impact. Using high-throughput, untargeted analytical methods that are up to 1,000 times faster than traditional approaches, we have enriched the ability to identify and validate key biomarkers that will enable sponsors to confidently employ clinical trial enrichment strategies, across more studies, more quickly.