The Right Treatment, the Right Time: Biomarker-Guided Assessment of Clinical Trial Dynamics
Adaptive clinical trial designs seek to add flexibility to the fixed, linear “design-conduct-analyze” sequence of conventional clinic trials. Instead of assessing results after the trial is completed, adaptive designs allow for interim data reviews so that adjustments can be made during the study if warranted.
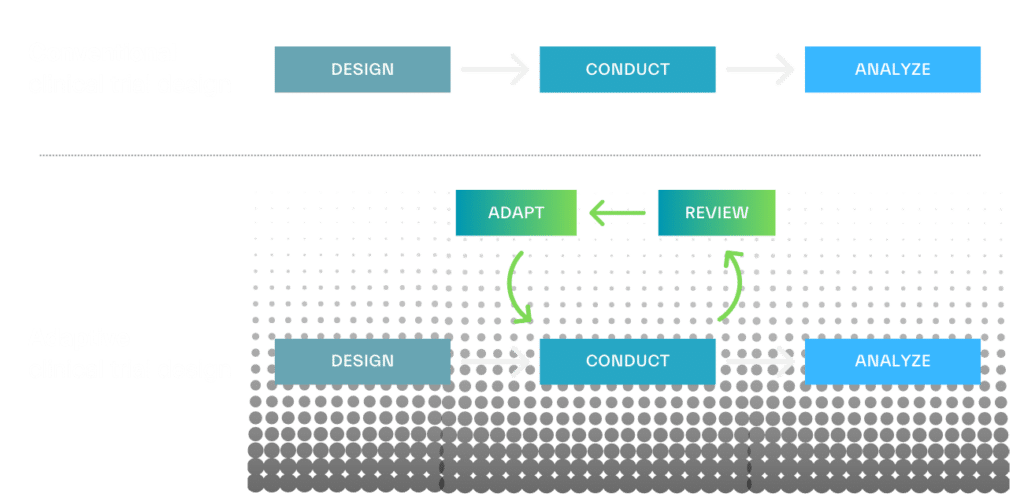
From refining sample size to adjusting dosing regimens, pre-defined trial components can be modified based on accumulating data to improve study efficiency. Adaptive designs can also head off ineffective trials before extensive time and cost is invested and decrease patient exposure to inefficacious therapies. This approach has the potential to drive smaller, shorter clinical trials and improve the likelihood of clinically relevant results.
Intervening in time based on biomarker insights
Biomarkers of response can play a critical role in enabling adaptive clinical trial designs, as they not only help to enroll patients most likely to benefit from the given treatment but can also be used to measure drug treatment effects in those patients throughout the trial, including clinical efficacy and toxicity responses.
Small molecule biomarkers are particularly well-suited for this application for several reasons. They are the most dynamic measures of biological processes and treatment response, and can therefore provide real-time information on the efficacy and safety of an intervention. Changes to a patient’s metabolome during the clinical trial can be detected early and may inform changes to their dosage, dosing schedule, or overall treatment protocol. Small molecule biomarkers can also readily traverse between tissue beds and blood so can often be captured via venipuncture. This provides a relatively non-invasive and cost-effective means for serial sampling and can support more frequent sampling over the course of the trial.
Case in point: Adapting a clinical trial of combination therapy
Let’s use an example of a biomarker-guided clinical trial testing two anti-cancer drugs for a solid tumor. The first question to address is which drug, or combination of drugs, each patient should initially receive. Biomarkers can be identified at baseline that indicate individuals who are likely to be responsive to each specific treatment, and those response biomarkers can be measured in each patient at enrollment to determine their optimal therapeutic regimen. For example, one patient’s biomarker profile may indicate they are most likely to respond to anti-cancer drug A, while another patient’s profile indicates responsiveness to anti-cancer drug B.
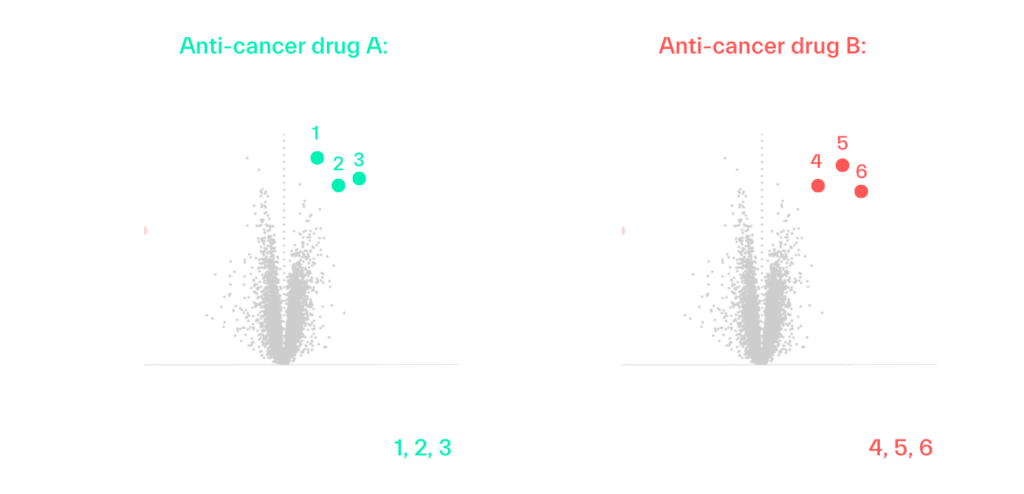
Predictive biomarkers can be used to enrich a conventional clinical trial at enrollment, but they of course do not guarantee a favorable outcome for each patient. This is where adaptive clinical trial designs can potentially lead to greater efficacy and trial efficiency, as those biomarkers can also be monitored through the trial to understand if and how the patient is truly responding to treatment, and how interventions should be adjusted.
With interval profiling of patients’ small molecule biomarker signatures, we can obtain real-time readouts of different aspects of their underlying tumor composition and its sensitivity to each drug. These measures may indicate that the tumor has responded well to initial treatment with anti-cancer drug A, but now shows the propensity for an elevated response to anti-cancer drug B. As a tumor changes with treatment, the therapeutic combination and dosing strategy can be changed as well.
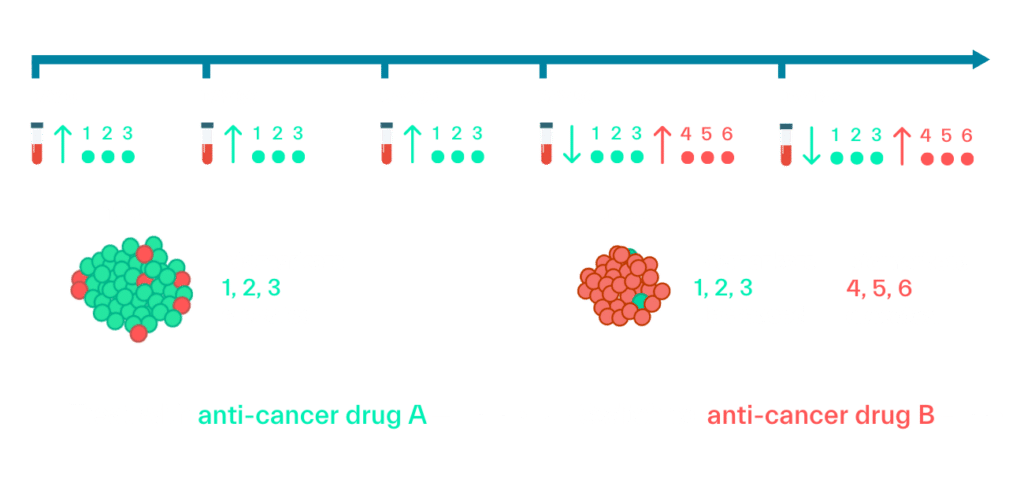
Frequent sampling and biomarker analysis can also help to identify patients who are non-responsive to treatment earlier in the trial, so higher dosages could be tested or those individuals can be removed from the study to limit unnecessary drug exposure.
The same discovery metabolomics approach used to capture biomarkers of treatment efficacy can also be applied to identify safety biomarkers for each therapy, with targeted profiling over the course of the trial to monitor for adverse events.
Equipped with these continual learnings, sponsors can monitor individual benefit-risk, modify trial arms to include subgroups with the most favorable response, reduce clinical trial size, and enable adaptive therapy to address the dynamics of complex disease.
Optimizing dosing and timing of treatments – and overall trial success
Small molecule biomarkers help support timely intervention to introduce, remove, increase, or combine therapies, and ultimately optimize trial outcomes. By applying these markers in an adaptive clinical trial design, ineffective treatments can be discontinued sooner and dose-response and dose-toxicity relationships in individual patients can be better understood to optimize dosing regimens. This approach can be applied to therapies individually or as combinations. The goal is to drive more efficient and informative clinical trials that require less patients but drive better outcomes with personalized therapies.
Sapient is ideally suited to support the discovery of baseline biomarkers of response and safety because of our high throughput, nontargeted technologies. We can screen samples from hundreds or thousands of people at a time, looking across broad chemistries to identify predictive biomarkers (whether known or unknown) of response to similar therapies. We can also reference our Human Biology Database, comprised of data from >100,000 samples from diverse individuals, to validate these predictive markers, and use our follow-on technology pipeline of traditional mass spectrometry systems for targeted screening of identified biomarkers in samples collected throughout the trial.
If you are interested in learning how Sapient can support your clinical trial strategies with high-throughput biomarker discovery as a service, feel free to reach out to us here.